Over a decade of research by St Catharine’s scientists based at the Cambridge Institute for Medical Research (CIMR) has culminated in the discovery of an entirely new mechanism for a rare genetic disease.
Published in Science Advances yesterday, the findings focus on alpha-1 antitrypsin (AAT), a protein made in the liver that normally protects our lungs from damage. Around 12,000 people in the UK suffer from alpha-1 antitrypsin deficiency, an inherited disorder in which abnormal forms of AAT accumulate in cells. This accumulation causes life-limiting and life-changing problems in the lungs and liver, including pulmonary emphysema, paediatric hepatitis and adult cirrhosis.
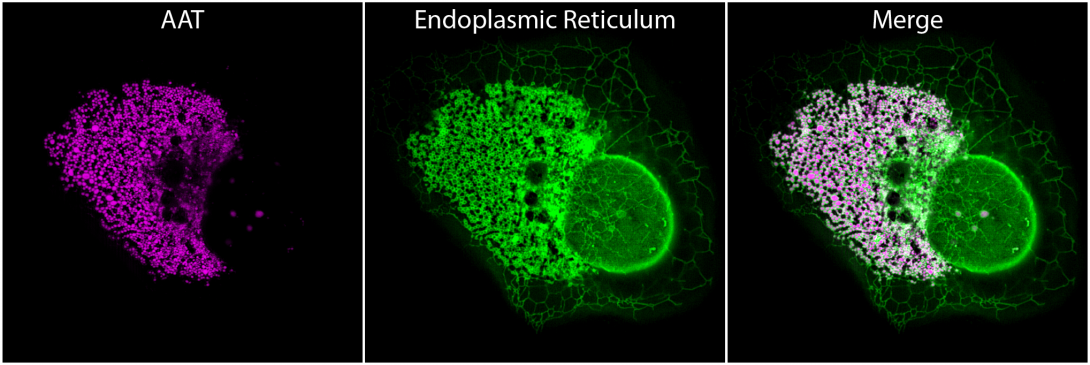
Working with colleagues from around the world, the CIMR team studied cells grown in the laboratory that were struggling to produce AAT, just like in the livers of people with AAT deficiency. They showed not only that abnormal forms of AAT clump together inside a crucial part of the cell called the endoplasmic reticulum – which ordinarily serves as an ‘assembly line’ for many of the different proteins that our cells produce – but also that these clumps act like a molecular ‘sieve’ and trap other larger endoplasmic reticulum proteins.
St Catharine’s Fellow Professor Stefan Marciniak (2011) is joint senior author of the new study with Dr Joseph Chambers, who was a postdoctoral associate at the College during 2017–20 when this exciting research began. Joint first author with Dr Chambers is Nikita Zubkov, who arrived at the Marciniak laboratory and St Catharine’s as an MPhil student in 2018 and has remained to complete his PhD. Max Schwiening, another St Catharine’s PhD student based at the CIMR, is also part of the research team and co-author on the paper.
Dr Chambers commented, “It was already known that abnormal AAT gets stuck inside liver cells and cannot be released to where it is needed. Our discovery of this mechanism of molecular sieving provides a new explanation for how liver cells are damaged in AAT deficiency, and paves the way for new therapies to protect the liver. Molecular sieving may also feature in other diseases, and we are excited by Nikita’s next phase of research looking more closely at this mechanism in dementia.”
Nikita said, “Our team has been fortunate to collaborate with other world-leading laboratories, enabling us to combine cutting-edge biophysical techniques and delve ever deeper into AAT deficiency in this research. This collaborative approach will stand us in good stead now we are starting to apply these techniques to other diseases.”
Professor Marciniak added, “My laboratory has been studying AAT deficiency for over ten years to advance our understanding of this devastating disease. I have been so impressed by the way that Joe and Nikita orchestrated this latest research and tapped into expertise from around the world. Nikita should also be particularly proud of the outstanding quality of his imaging work, which underpins our paper. I look forward to seeing the outcomes of his final PhD experiments and what these reveal about other diseases.”
This pioneering research has been supported by three 2-year grants from the Alpha-1 Foundation, one of which funded Nikita’s PhD, and a further generous grant of £469,880 from the Medical Research Council in 2021.
Reference
Chambers J, et al. Z-α1-antitrypsin polymers impose molecular filtration in the endoplasmic reticulum after undergoing phase transition to a solid state. Science Advances 2022; 8 (14). https://www.science.org/doi/10.1126/sciadv.abm2094