St Catharine’s Fellow Dr Andrzej Harris is part of an international team of scientists that has used advanced microscopic techniques to take a close-up look at a protein that enables invasive fungal infections to resist treatment. Their findings were recently published by Nature Communications and could pave the way for new approaches in response to the threat of antimicrobial resistance.
Antimicrobial resistance is one of the top ten global public health threats facing humanity, according to the World Health Organization. With only a few antifungal drugs available, it can be especially dangerous when a fungal infection does not respond to treatment (known as antifungal resistance).
Invasive fungal infections are rapidly life threatening and a particular concern for patients who are immunocompromised because of conditions such as cancer or HIV. Most recently, fungal infections have been identified as a complication for severe and critically ill patients with COVID-19.
Dr Harris, who is based at the University of Cambridge's Department of Biochemistry, explains, “While misuse and overuse of antimicrobial medicines are major factors in the spread of antimicrobial resistance, fungal infections can also resist treatment due to a group of proteins that occur naturally in cell membranes, which expel drug molecules from cells to prevent them from taking effect.”
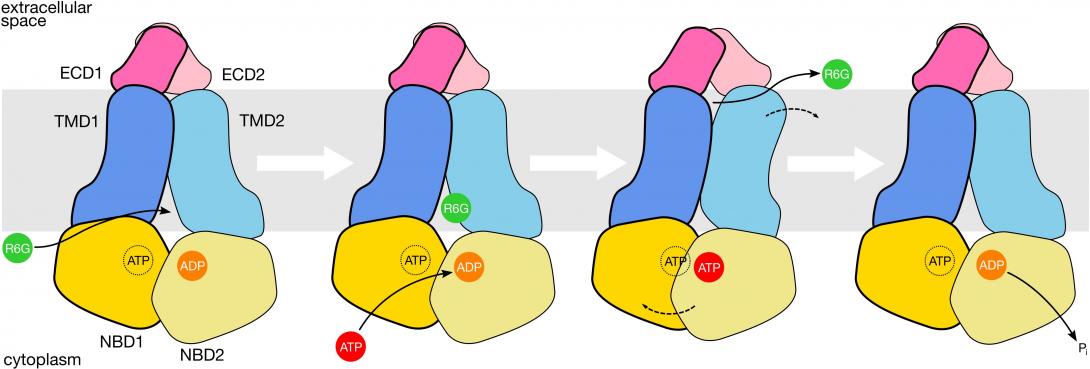
Pleiotropic drug resistance protein 5 (Pdr5) is one of these proteins and has been widely studied since its discovery in brewer’s yeast in 1990. It is now known to confer resistance to a vast range of different drugs, although relatively little has been understood about its structure.
The research utilises the state-of-the-art technique of electron cryo-microscopy, or cryo-EM, which can visualise proteins at the resolution of individual atoms and even show antibiotic molecules inside the protein.
Dr Harris adds, “Despite three decades of extensive study of Pdr5 biochemistry, the protein has evaded structural characterisation. Using cryo-EM, we have been able to reveal detailed information not only about the molecular structure of Pdr5, but also how it transmits drugs out of cells before they can harm the fungus. I am excited that our research might open up new avenues for the development of effective antifungal compounds.”
Reference
Harris, A et al. Structure and efflux mechanism of the yeast pleiotropic drug resistance transporter Pdr5. Nature Communications 2021; 12: 5254. Available online: https://www.nature.com/articles/s41467-021-25574-8